We use acids, and bases on every day of our lives, and these substances are also incredibly useful in biology and medicine. Our everyday diets contain acidic foods lemon juice, limes, grapes, pomegranates, grapefruits, blueberries, pineapples, vinegar, tamarind, curd and basic foods Almonds. Spinach, Garlic, Broccoli, Basil, Red Onion, Baking soda etc. Gastric Hydrochloric acid secretion playing major role for digestion of foods.

ALKALINE FOOD ACIDIC FOOD https://fbcdn-sphotos-c-a.akamaihd.net/hphotos-ak-ash4/313857_553435718022545_666046031_n.jpg |
Acid (Acidus) word is derived from latin word ‘acer’ meaning sour to taste and bases or alkali (ash of wood means alkalai in Arabic, alquili in latin) bitter in taste.
Older concept of Acid and Base
An acid is a substance whose aqueous solution -i) sour in taste ii) turns blue litmus red iii) neutralizes alkali iv) reacts with metals and liberates hydrogen v) reacts with carbonate, bicarbonate and releases CO2.
An base is a substance whose aqueous solution -i) bitter in taste ii) turns red litmus blue iii) neutralizes acids and iv) gives a soapy solution.
Theories of Acids and bases
The definition of acids and bases can be done within the framework of many theories and some important theories are described belows.
Arhenius theory
Svante Arrhenius (1884), According to this theory, an acid is a substance, which gives hydrogen ions (H+, proton) in aqueous solution whereas base is a substance which gives hydroxyl ions (OH–) in aqueous solution.

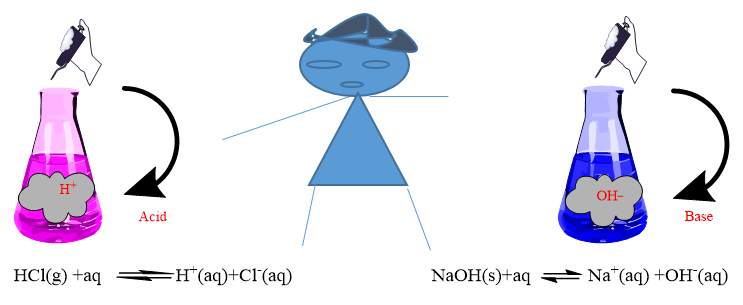
Example-
Acids: HCl, HNO3, H2SO4, HCN, Acetic acid (vinegar), oxalic acid (tomato), citric acid (citrus fruit), formic acid (Ant sting), Maleic Acid (unripe green apple), Tannic acid (tea), Tartaric acid (tamarind), Lactic acid (curd).
Bases: NaOH (caustic Soda), KOH (Caustic Potash), Mg(OH)2, NH4OH, Ca(OH)2 (slaked lime).
According to these theory a neutralisation reaction taking place between combination of an acid and bases.
ACID + BASE → WATER + SALT
Limitation-
*It fails to explain acidic and basic character of a substance in solvents other than water (alcohol, liquid ammonia)
*The neutralisation of acid and base in absence of solvent could not be explained.
*This theory only explain in terms of aqueous solution but not in terms of substance.
*Fail to explain the acidic nature of CO2, SO2, AlCL3 and basic nature of CaO, MgO, NH3
In 1923 Johannes Bronsted (Copenhagen) and Thomas M. Lowry (England) proposed this theory.
According to this theory an acid is a substance (molecule or ion) that can donate a proton (proton donor) to any other substance, whereas base is a substance that can accept a proton (proton Acceptor) from any other substance.
Acids are proton donor and bases are proton acceptor so popularly this theory known as proton donor acceptor theory.
Acids –HF, HCl, HNO3, H2SO4, H2S, CH3COOH, H2O, H3PO4 (Molecule)
H3O+, NH4+, HSO4–, HCO3 – (ion)
Bases -NH3, H2O, RNH2, R2NH (Molecule)
OH–, S2–, CO32-, Cl–, Br–, NO3– etc. (ion)

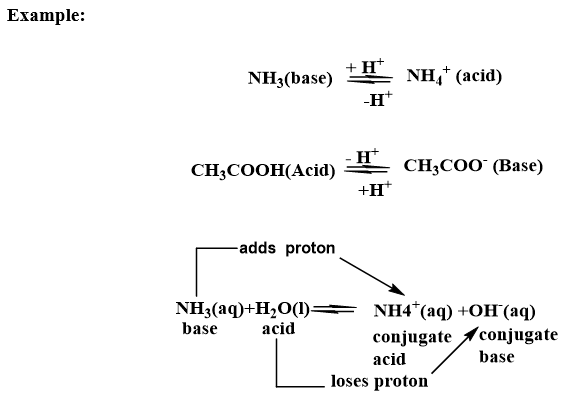
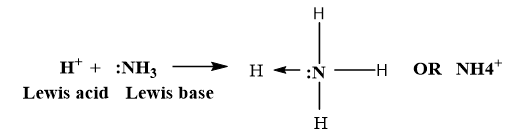
In dissolution of Ammonia in water, the water and ammonia are acting as proton donor and acceptor respectively and are Bronsted-Lowry acid and base.
The pair of substances that differ from one another by a proton are called conjugate acid-base pairs.
Here, OH– is the conjugate base of acid H2O and NH4+ is conjugate acid of base Ammonia (NH3).
Another example, Ionization of HCL in H2O
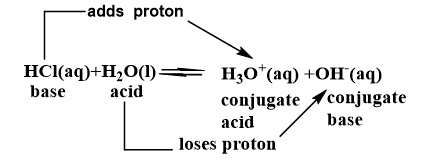
Stronger the Bronsted acid weaker it’s conjugate base and Vice versa.
Conjugate acid has one more proton than acid and conjugate base has one less proton than acid.
All Arrhenius acids are Bronsted Acids but all Bronsted bases may not be Arrhenius Bases.
Limitation
*The substance those does not contain Hydrogen atom although they are acidic
Example-CO2, SO2, NO2, ALCl3, FeCl3, BF3
*This concept only emphasis on proton transfer
*A large number of reaction acid-base reaction there is no proton transfer take place.
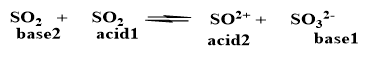
*Fails to explain the reaction between acidic oxides such as CO2, SO2 and basic oxides such as CaO, BaO.
Lewis theory (Electron acceptor donor theory)
This theory is known as modern theory of acid and base in terms of electron.
In 1923, G.N.Lewis defined an acid is a substance (atom, molecule, or ion) which accept pair of electron whereas base is a substance which donate pair of electron.

An acid is an electron pair acceptor and base is and electron pair donor.so popularly known as Electron donor-acceptor theory.
An acid is an electron deficient species and base is a electron rich species
An electrophile (electron lover) and all positive charge-LEWIS ACID
A Nucleophile (nucleus Lover) and all negative charge- LEWIS BASE
All positive charge
Example
Lewis acid– AlCl3, FeCl3, FeBr3 (neutral),
Fe3+,H+, H3O+, Al3+,Mg2+(positive Charge)
Lewis Base- H20, RNH2, NH3 (neutral)
CN–, OH–, Cl–, Br–, F– , S2-etc (negative charge)
Acid base neutralisation reaction takes place by sharing of electron pair of base to an acid and form a coordinate bond (dative bond).
Limitation
*It fails to explain the acidic character of
common acid like HCl, HNO3 which donot form coordination bond
*It does not provide the information about
relative strength of acid and base.
*As acid base reactions involve
electron they are expected to fast but
due to formation of coordination bond it is relatively slow.
Strong
Base and weak Base
The base which completely ionised in
aqueous solution is called strong base and those are weakly ionised are weak
base.
Example-
NaOH,
KOH (strong base) NH4OH, AgOH, Ca (OH)2 (weak base)
Hard
and soft acid –base concept (HSAB):
According
to Pearson’s, hard acid are electron acceptor those
having high positive charge small size and empty valance cell orbital.
Whereas, soft acid have low positive
charge, large size and field valance cell orbital.
Example
Hard
acid: H+, Li+, Na+,
K+, Mg+, Ca2+, Sr2+, Al3+, Fe3+, Sn4+, BF3 etc.
Softacid:
Cu+,
Ag+, Au+, Hg+, Cd2+, I+, Br+, I2+, Br2+, O, Cl, Br, I etc.
Relative
Strength of Acids and Bases
The greater the no of H+ ions
produced in the aqueous solution, stronger is the acid, and also greater the no
of OH– ions produced in the aqueous solution, stronger is the base.
Strong
acid and weak Acid-T he acid which completely ionised and
produce large number of H+ in aqueous
solution is called strong acid and those are weakly ionised give lesser number
of H+ ion are weak acid
Example- HCl,
H2SO4, HNO3 (strong acid) CH3COOH, HCN,
HF, (COOH)2 (weak acid)

Hi…! Currently, I am working as an Professor at Department of Pharmaceutical Chemistry(H.O.D),The Pharmaceutical College, Barpali, Odisha. I have more than 19 years of teaching & research experience in the field of Chemistry & Pharmaceutical sciences.
