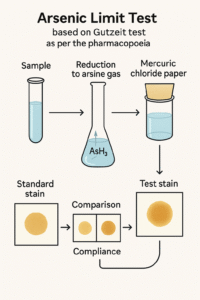
 The pharmacopoeial limit test of arsenic is a qualitative comparative test bsed on Gutzeit test in which arsenic impurity present in the sample is reduced to arsine gas and then it is passed through on mercuric chloride paper as a yellow to brown stain, Which is compared against prepared standard stain as prescribe by Pharmacopoiea.If the sample stain is not deeper than standard stain ensuring the substance complies with the prescribed arsenic limit.
The pharmacopoeial limit test of arsenic is a qualitative comparative test bsed on Gutzeit test in which arsenic impurity present in the sample is reduced to arsine gas and then it is passed through on mercuric chloride paper as a yellow to brown stain, Which is compared against prepared standard stain as prescribe by Pharmacopoiea.If the sample stain is not deeper than standard stain ensuring the substance complies with the prescribed arsenic limit.
Reason and scope
Pharmacopoeias require a limit test rather than an assay to make sure that any arsenic present does not exceed a very low limit, which is usually expressed in parts per million (ppm) for the monograph in the matter. This is because arsenic, even in trace amounts, is an unwanted impurity in pharmaceutical substances due to its toxicity. A visual comparison of the intensity of a colored stain produced by arsine on mercuric chloride paper with a standard stain produced under the same conditions from a known small amount of arsenic serves as the end point for the limit test, providing an easy and reliable compliance check in quality control.
Principle
The Arsenic impurities present in the sample is reduced by nascent hydrogen (from zinc and acid) to arsine gas, which then reacts with mercuric chloride paper to form a colored mercuric arsenide stain whose intensity is proportional to the amount of arsenic present. This test is based on a series of redox transformations. In daylight, the test stain is compared with a standard stain that was made simultaneously from a standard arsenic solution with a specified concentration, (usually 10 ppm). As in the test conditions, if the intensity of test stain is not deeper than standard , then compliance is confirmed.
Reactions
- Reduction of pentavalent arsenic to trivalent arsenic by stannous species:
H3AsO4+H2SnO2 → H3AsO3+H2SnO3 - Reduction of arsenious acid to arsine by nascent hydrogen generated in situ:
H3AsO3+3 H2 →AsH3↑+ 3 H2O - Reaction of arsine with mercuric chloride paper producing yellow to brown mercuric arsenide
2AsH₃ + HgCl₂ → Hg(AsH₂)₂ + 2HCl
Reagents and their roles
- Stannated hydrochloric acid: provides chloride medium and stannous species to ensure complete reduction of any pentavalent arsenic to trivalent arsenious acid .
- Zinc (granulated, arsenic-free, denoted “AsT”): reacts with acid to generate a steady stream of nascent hydrogen that reduces arsenious acid to arsine gas efficiently.
- Potassium iodide: accelerates reduction of higher-valent arsenic and stabilizes the reduction environment, supporting quantitative arsine evolution .
- Lead acetate cotton plug: traps any hydrogen sulfide evolved so it does not reach the mercuric chloride paper and cause spurious darkening, maintaining specificity of the stain to arsine.
- Mercuric chloride paper: the indicator surface that forms a yellow/brown mercuric arsenide stain proportional to arsine exposure. It is used for the visual comparison.
All reagents designated for this test should be certified “arsenic-free” or “AsT” grade to avoid reagent-borne background that could increase the test stain intensity and yield false failures.
Apparatus
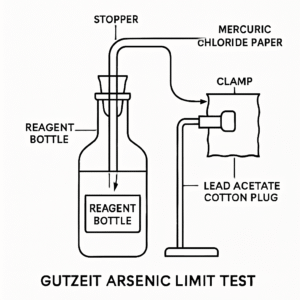
A Gutzeit arsenic apparatus consists of a suitable reaction bottle or conical flask closed by a stopper through which passes a glass tube assembly holding, from bottom to top, a lead acetate cotton plug and, at the top interface between two flat tube ends, a square of mercuric chloride paper clamped between the tube faces to intercept the ascending gas stream. The design ensures that the hydrogen/arsine stream passes through the lead acetate trap into contact with the mercuric chloride paper at a fixed distance and geometry, producing a stain that can be compared reproducibly with a standard run side-by-side under identical conditions for a fixed time (commonly 40 minutes).
 Standard solution and sensitivity
Standard solution and sensitivity
Pharmacopoeial-style procedures commonly employ a standard arsenic solution corresponding to a defined ppm concentration; many educational and institutional protocols describe a 10 ppm As standard for comparison, prepared from arsenic trioxide by alkali dissolution and subsequent dilutions under prescribed steps. One such preparation described in instructional resources dissolves 0.330 g of arsenic trioxide in 2 M sodium hydroxide, dilutes to 250 mL, and then a defined aliquot is further diluted 1:100 to yield a convenient standard strength, with final comparison conducted using 1 mL of this standard diluted to 50 mL in the apparatus during the test period.
Stepwise procedure
- Prepare two Gutzeit assemblies labeled Standard and Test, inserting loosely packed lead acetate cotton in the lower tube and fixing a fresh square of mercuric chloride paper between the flat tube faces under the spring clips, ensuring gas must traverse the 6.5 mm to 8 mm inner passage area to contact the paper.
- Standard: Place 50 mL of water in the reaction bottle, add 1 mL of the arsenic standard solution (e.g., 10 ppm As), then add 10 mL of stannated hydrochloric acid, 1 g potassium iodide, and 10 g of granulated zinc, assemble immediately, and allow to stand for 40 minutes away from direct light.
- Test: Dissolve the specified test quantity of the sample in 50 mL of water in the matching apparatus bottle, add 10 mL of stannated hydrochloric acid, 1 g potassium iodide, and 10 g of arsenic-free granulated zinc, assemble immediately, and allow to stand for 40 minutes under the same conditions as the standard.
- After the exposure period, compare the yellow/brown stain on the mercuric chloride paper of the Test with that of the Standard in daylight without delay; compliance is affirmed if the Test stain is no more intense than the Standard stain.
Observations and interpretation
The intensity of yellow to brown coloration on the mercuric chloride paper increases with the amount of arsine generated, which reflects the arsenic content of the sample under the fixed conditions of reagent amounts. If, upon immediate daylight comparison at 40 minutes, the Test stain is lighter than or equal to the Standard stain, the material passes the limit test for arsenic; a darker Test stain indicates non-compliance relative to the specified limit.
Reaction timing and conditions
A 40-minute evolution period is specified in many procedural descriptions to standardize arsine generation and adsorption, minimizing variability associated with slower kinetics or paper saturation and thereby improving comparability between Test and Standard. Performing the exposure in the dark or away from bright light and comparing immediately in daylight helps stabilize the stain development and avoids photodegradation or continued reaction beyond the assay window that could skew interpretation.
Roles of individual reagents
- Stannous chloride, typically in hydrochloric acid, is emphasized as ensuring complete evolution of arsine by first reducing As(V) to As(III), safeguarding the uniformity of arsine generation across samples that might contain arsenic in different oxidation states.
- Zinc’s granulated form increases surface area for hydrogen evolution and, together with acid, is the key reductant forming the nascent hydrogen required for the As(III) to AsH3 step under the mild, wet conditions of the test.
- Potassium iodide both accelerates and stabilizes the reduction system and is part of the traditional Gutzeit protocol specified in instructional and compendial-style writeups, contributing to the reproducibility of the stain within the time limit.
Interferences and controls
Hydrogen sulfide, if formed from sample matrices or impurities, can darken the mercuric chloride paper independently of arsenic, hence the incorporation of a lead acetate cotton trap to capture sulfide before it reaches the indicator paper. All reagents must themselves be low in arsenic (AsT), and test glassware and water quality must be carefully controlled to avoid background staining that would invalidate the comparison or raise the baseline of the Test and Standard alike.
Alternative indicator papers
Most pharmacopoeial and instructional protocols specify mercuric chloride paper; however, some teaching sources note that mercuric bromide paper has been used for analogous arsine tests, though chloride paper remains the standard in British Pharmacopoeia-style descriptions for the pharmaceutical limit test context. Choice of paper affects stain hue and sensitivity, and any alternative must be validated to ensure equivalence in intensity response to the standard arsenic exposure under the procedure’s 40-minute window.
Apparatus design details
A representative apparatus features a 100 mL bottle (or conical flask) stoppered with a through-tube whose lower tip is narrowed to about 1 mm internal diameter with a lateral orifice 2–3 mm in diameter 15 mm from the tip, and an upper flat tube end clamping the mercuric chloride paper against a matching flat surface of a short tube held by springs, ensuring consistent gas flow and paper contact. The upper end of the tube should be flat and perpendicular to the axis to hold the paper evenly, and the lateral orifice depth relative to the stopper ensures proper gas distribution across the lead acetate region before reaching the indicator.
Typical calculations and ppm context
When using a 10 ppm As standard, the comparison embodies the concept that the Test solution’s arsenic contribution during the 40-minute exposure should not produce a stain deeper than the stain produced by the known 10 ppm standard under identical conditions, thereby providing a practical pass/fail at the indicated monograph limit. The pharmacopoeial monographs state the actual limit applicable to each substance, and the side-by-side comparison operationalizes that limit without numerical calculation in routine QC practice.
Safety notes
Arsine (AsH3) is highly toxic, so the evolution should be performed in a well-ventilated hood and personnel should minimize exposure, handle reagents carefully, and dispose of mercuric indicator papers as hazardous waste per institutional guidelines; occupational exposure limits for arsenic emphasize the need for strict controls in laboratory environments. Lead acetate components and mercuric chloride paper also present toxicity hazards, reinforcing the requirement for appropriate personal protective equipment and waste handling practices in accordance with laboratory safety standards.
care should be taken.
- Weak or no stain in the Standard may indicate exhausted or contaminated mercuric chloride paper or incorrect standard preparation; fresh paper and verified standard preparation should restore the expected faint but distinct stain at 40 minutes.
- Excessively dark stains in both Test and Standard can result from contaminated reagents or apparatus; repeating with certified AsT reagents and cleaned apparatus is necessary to re-establish a reliable baseline.
- Smearing or irregular stain edges can result from improper clamping or uneven paper surfaces; ensuring flat contact between tube faces and square, undamaged paper improves consistency.
Notes on method variants
While the classical Gutzeit test uses mercuric chloride paper, some descriptions note mercuric bromide indicator use in related contexts, but standard pharmaceutical limit tests remain aligned with mercuric chloride paper and 40-minute comparisons to maintain compendial continuity and reproducibility. Minor procedural variations in reagent quantities exist among institutional protocols, but the essential chemistry and the comparative end point are conserved across authoritative renditions.
Limitations
This is a semi-quantitative, comparative method and does not yield a numeric arsenic content, but rather a pass/fail outcome at the specified limit, requiring careful matching of conditions between Standard and Test for validity. The method’s reliance on human visual comparison necessitates good lighting, immediate evaluation, and experienced operators to minimize subjective variation, though the stark intensity difference at compliance thresholds usually provides adequate discrimination.
Key Points and Summary
Arsenic Limit Test (Gutzeit Method)
- Principle: Arsenic in the sample is reduced to arsine gas, which passes through mercuric chloride paper, producing a yellow/brown stain compared to a standard stain; compliance is confirmed if the test stain is not deeper than the standard.
- Purpose: Ensures arsenic impurity is below pharmacopeial limits, typically expressed in ppm, because even traces are toxic.
- Reactions: As(V) is reduced to As(III), then arsine (AsH₃) is formed and reacts with HgCl₂paper:
H3AsO4+H2SnO2→H3AsO3+H2SnO3
H3AsO3+3 H2→AsH3+3 H2O
2AsH3+HgCl2→Hg(AsH2)2+2HCl2 - Key reagents: Stannated hydrochloric acid (reduces As (V)), zinc (nascent hydrogen, AsH₃ formation), potassium iodide (accelerates reduction), lead acetate cotton (removes sulfides), mercuric chloride paper (detects arsine).
- Apparatus: Gutzeit bottle with lead acetate plug and plunger for HgCl₂ paper, run for 40 min side-by-side with standard.
- Interpretation: Test passes if stain intensity is less than or equal to the standard’s; quick daylight comparison at 40 min.
- Safety: Handle AsH₃, HgCl₂, and lead acetate with care; use in fume hood.
- Troubleshooting: Use fresh reagents, clean apparatus, and proper paper placement to avoid false results.
References
- Chatwal GR, Pharmaceutical Inorganic Chemistry
- Block JH, Roche EB, Soine TO, Wilson CO, Inorganic Medicinal and Pharmaceutical Chemistry, Lea & Febiger, 1974
- Indian Pharmacopeia 1985

Hi…! Currently, I am working as an Professor at Department of Pharmaceutical Chemistry(H.O.D),The Pharmaceutical College, Barpali, Odisha. I have more than 19 years of teaching & research experience in the field of Chemistry & Pharmaceutical sciences.

Nice content
Thanks for your comments.hoping the best