What are Organic Compounds?
The compounds Which are generally derived or obtained from living organisms (plants,animals and microorganism) are called Organic compounds. whereas “inorganic” compounds are obtained from non-living sources (rock, salts,minerals,sea sources, As the organic compounds mostly contain carbon so it is is also called cabon compounds.
The branch of chemistry which deals with the study of organic compounds, their structure, properties, composition, reactions, and synthesis is called Organic chemistry. And also these branch popularly known as carbon chemistry. Today, organic chemistry generating millions of compounds, both naturally occurring and synthetically produced, making it one of the most extensive and dynamic fields of chemistry.
Why the properties of carbon is unique?
Carbon has four valence electron which can able to form four covalent bond with same and different atoms. (Tetra covalency).
Carbon has self-linking properties (Catenation), so it can able to form straight chain, branched chain and cyclic organic compounds.
Carbon can intermix its s,p orbital undergoes sp3, sp2 and sp hybridization (intermixing of orbital) to form C-C,C=C, C≡C respectively and form possible wide variety of
Additionally, carbon can form stable bonds with hydrogen, oxygen, nitrogen, sulfur, phosphorus, and halogens, and create so many organic molecules.
Historical Development of Organic Chemistry
Early Concepts and Vital Force Theory
The history of organic chemistry can be traced back to ancient civilizations that extracted and utilized natural products such as dyes, perfumes, medicines, and fermented beverages. However, systematic study began in late 18th and early 19th centuries.
During this period, chemists observed a fundamental distinction between substances derived from living organisms (organic) and those obtained from minerals (inorganic). The prevailing theory, known as Vital Force Theory, proposed that organic compounds possessed a special “vital force” present only in living organisms and could not be synthesized in the laboratory from inorganic materials. This belief was deeply rooted in philosophical and scientific thought of the time.
Failure of Vital Force Theory: Wöhler’s Urea Synthesis
A watershed moment in organic chemistry occurred in 1828 when the German chemist Friedrich Wöhler successfully synthesized urea (NH₂CONH₂), an organic compound found in urine, from ammonium cyanate (NH₄OCN), an inorganic salt. By simply heating ammonium cyanate, Wöhler obtained urea
NH₄OCN ——–> NH₂CONH₂
demonstrating that organic compounds could be produced artificially without the intervention of living organisms.
This groundbreaking experiment shattered the vitalism theory and opened the door to synthetic organic chemistry. Wöhler’s synthesis is considered one of the most significant milestones in the history of chemistry, fundamentally changing the way scientists understood the nature of organic compounds.
Structural Theory and the Benzene Ring
Following Wöhler’s discovery, chemists turned their attention to understanding the structure of organic molecules. In the mid-19th century, several scientists, including August Kekulé, Archibald Scott Couper, and Alexander Butlerov, developed the structural theory of organic chemistry. This theory proposed that atoms in molecules are connected in specific arrangements, and the properties of compounds depend on these structural patterns.
Kekulé’s benzene structure (1865) was particularly revolutionary. He proposed that benzene (C₆H₆) has a cyclic structure with alternating single and double bonds, resembling a hexagonal ring. This insight laid the foundation for understanding aromatic compounds and their unique stability, a concept central to modern organic chemistry.
Development of Reaction Mechanisms
In the 20th century, organic chemistry evolved from merely describing structures to understanding reaction mechanisms—the step-by-step processes by which chemical reactions occur. Pioneers such as Christopher Ingold, Robert Robinson, and Linus Pauling introduced concepts like nucleophiles, electrophiles, resonance, and hybridization, providing a theoretical framework for predicting and explaining organic reactions.
The advent of spectroscopic techniques (NMR, IR, UV-Vis, mass spectrometry) further revolutionized the field, allowing chemists to elucidate molecular structures with precision and monitor reaction progress in real-time.
Modern Organic Chemistry
Today, organic chemistry is an interdisciplinary science intersecting with biochemistry, medicinal chemistry, materials science, and nanotechnology. Advances in computational chemistry and green chemistry principles continue to shape the field, emphasizing sustainability and efficiency in chemical synthesis.
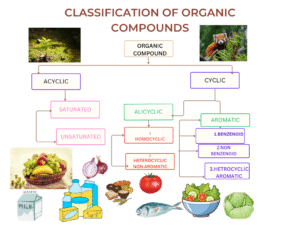
Classification of Organic Compounds
Organic compounds can be classified in multiple ways based on their structure, functional groups, and chemical behavior,
Based on Carbon Skeleton
Acyclic or Open-Chain Compounds (Aliphatic Compounds)
These compounds consist of carbon atoms arranged in straight or branched chains without forming rings. They are further subdivided into:
- Saturated Hydrocarbons (Alkanes): Contain only single bonds between carbon atoms (e.g., methane, ethane, propane). The general formula is CₙH₂ₙ₊₂.
- Unsaturated Hydrocarbons: Contain one or more double or triple bonds.
- Alkenes: Contain at least one carbon-carbon double bond (C=C), with the general formula CₙH₂ₙ (e.g., ethene, propene).
- Alkynes: Contain at least one carbon-carbon triple bond (C≡C), with the general formula CₙH₂ₙ₋₂ (e.g., ethyne, propyne).
Cyclic Compounds
These compounds have carbon atoms arranged in closed ring structures. They are divided into:
- Alicyclic Compounds: Cyclic compounds that resemble aliphatic compounds in properties (e.g., cyclopropane, cyclohexane).
- Aromatic Compounds: Contain one or more benzene rings or similar structures exhibiting aromaticity—a special stability due to delocalized π-electrons. Examples include benzene, toluene, naphthalene, and anthracene. Aromaticity is governed by Hückel’s rule, which states that aromatic compounds must be planar, cyclic, fully conjugated, and contain (4n+2) π-electrons.
- Heterocyclic Compounds: Rings containing one or more heteroatoms (atoms other than carbon, such as nitrogen, oxygen, or sulfur). Examples include pyridine, furan, thiophene, and indole. These compounds are crucial in biological systems and pharmaceuticals.
Based on Functional Groups
Functional groups are specific groups of atoms within molecules responsible for characteristic chemical reactions. Classification based on functional groups is fundamental in organic chemistry:
- Alcohols (R-OH): Contain a hydroxyl group (e.g., methanol, ethanol).
- Ethers (R-O-R’): Contain an oxygen atom connected to two alkyl or aryl groups (e.g., diethyl ether).
- Aldehydes (R-CHO): Contain a carbonyl group (C=O) bonded to at least one hydrogen atom (e.g., formaldehyde, acetaldehyde).
- Ketones (R-CO-R’): Contain a carbonyl group bonded to two carbon atoms (e.g., acetone).
- Carboxylic Acids (R-COOH): Contain a carboxyl group (e.g., acetic acid, benzoic acid).
- Esters (R-COO-R’): Derived from carboxylic acids and alcohols (e.g., ethyl acetate).
- Amines (R-NH₂, R₂NH, R₃N): Contain nitrogen atoms bonded to alkyl or aryl groups (e.g., methylamine, aniline).
- Amides (R-CONH₂): Derived from carboxylic acids and amines (e.g., acetamide).
- Halides (R-X): Contain halogen atoms (F, Cl, Br, I) bonded to carbon (e.g., chloromethane, bromoethane).
- Nitriles (R-C≡N): Contain a cyano group (e.g., acetonitrile).
Homologous Series
A homologous series is a group of organic compounds with the same functional group and similar chemical properties, differing by a -CH₂- unit. Members of a homologous series show a gradation in physical properties such as boiling point, melting point, and density. Examples include the alkane series (methane, ethane, propane) and the alcohol series (methanol, ethanol, propanol).
Importance of Organic Compounds
Organic compounds are indispensable to life and modern civilization. Their significance spans biological, industrial, pharmaceutical, agricultural, and environmental domains.
Biological Importance
Life as we know it is based on organic chemistry. The major classes of biomolecules are all organic compounds:
- Carbohydrates: Serve as energy sources (glucose, starch) and structural components (cellulose in plants).
- Proteins: Composed of amino acids, they function as enzymes, hormones, antibodies, and structural elements.
- Lipids: Include fats, oils, phospholipids, and steroids, playing roles in energy storage, cell membrane structure, and signaling.
- Nucleic Acids: DNA and RNA carry genetic information and direct protein synthesis.
Metabolic pathways involve countless organic reactions catalyzed by enzymes, demonstrating the centrality of organic chemistry in biochemistry and molecular biology.
Pharmaceutical and Medicinal Importance
The development of drugs and therapeutic agents relies heavily on organic chemistry. Most pharmaceuticals are organic compounds designed to interact with biological targets such as enzymes, receptors, and nucleic acids. Examples include:
- Aspirin (acetylsalicylic acid): An analgesic and anti-inflammatory drug.
- Penicillin: An antibiotic that revolutionized medicine.
- Paclitaxel (Taxol): A chemotherapy drug derived from natural sources.
Medicinal chemists synthesize and modify organic molecules to enhance efficacy, reduce side effects, and overcome drug resistance, underscoring the importance of organic chemistry in healthcare.
Industrial Applications
Organic compounds are the basis of numerous industries:
- Polymers and Plastics: Synthetic polymers such as polyethylene, polypropylene, PVC, and polystyrene are derived from organic monomers and are ubiquitous in packaging, construction, textiles, and consumer goods.
- Dyes and Pigments: Organic dyes are used in textiles, printing, and cosmetics.
- Solvents: Organic solvents like acetone, ethanol, and toluene are essential in chemical manufacturing and laboratories.
- Fuels: Petroleum products (gasoline, diesel, kerosene) are mixtures of hydrocarbons providing energy for transportation and industry.
- Detergents and Surfactants: Organic molecules with hydrophobic and hydrophilic regions enable cleaning and emulsification.
Agricultural Applications
Organic chemistry contributes to agriculture through:
- Pesticides and Herbicides: Control pests and weeds, increasing crop yields.
- Fertilizers: Organic nitrogen compounds enhance soil fertility.
- Plant Growth Regulators: Hormones like auxins and gibberellins regulate plant development.
Environmental and Energy Applications
- Biofuels: Organic compounds such as ethanol and biodiesel offer renewable energy alternatives.
- Biodegradable Plastics: Designed to reduce environmental pollution.
- Green Chemistry: Emphasizes sustainable organic synthesis with minimal environmental impact.
Conclusion
Organic chemistry is an important branch of modern science. The classification of organic compounds based on structure and functional groups provides a systematic framework for studying their properties and reactions. The historical development of organic chemistry, from the fall of vitalism to modern synthetic methods, reflects the dynamic and evolving nature of the discipline.
The importance of organic compounds cannot be overstated—they are essential to biological systems, pharmaceuticals, industry, agriculture, and environmental sustainability. Mastery of organic chemistry requires not only memorization of facts but also a deep understanding of underlying principles and mechanisms
Hi…! Currently, I am working as an Professor at Department of Pharmaceutical Chemistry(H.O.D),The Pharmaceutical College, Barpali, Odisha. I have more than 19 years of teaching & research experience in the field of Chemistry & Pharmaceutical sciences.